SymbioCellTech's CSO Anna Gooch, PhD, to Present on Neo-Islet™ Technology at ADA 84th Scientific Sessions
Anna Gooch, PhD, will deliver an oral presentation on Neo-Islet™ technology at the American Diabetes Association's 84th Scientific Sessions on Sunday, June 23, 2024. Her presentation, titled "334-OR: Allogeneic Neo-Islets (NIs) Reduce Insulin Need by up to 1.2 U/kg bw in Autoimmune Type 1 Diabetes Dogs over Three Years without Requiring Antirejection Drugs, Enough to Meet Human Type 1 Diabetes Mellitus Needs," will include a Q&A session for attendees.
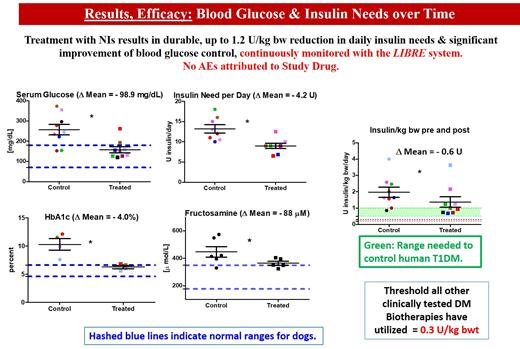
The spontaneously diabetic dog is ideal to test therapies intended for humans with T1DM. Neither insulin treated dogs nor humans spontaneously revert. Both have islet autoantibodies, shortened life expectancy, end-organ damage, while dogs need more insulin (humans: 0.5-1; dogs: 1-4 U/kg/day). We tested Neo Islets (NIs), 3-D organoids made of culture expanded islet cells and Mesenchymal Stem Cells in 9 spontaneously DM pet dogs. NI dose = 2x10e5 per kg bw, given i.p. to sedated dogs under ultrasound guidance. Follow up: Blood Glucose Levels monitored with continuous monitors. Insulin needs were recorded, and late follow-up levels of serum Creatinine, BUN and proteinuria were monitored. Each dog served as its own control. UA, full chemistries, lipids, CBC were assessed at baseline, monthly for 3 months, and biannually for 3 years. Results in Figure.
Allogeneic NI treatment is safe and effective (3 year follow up). T1DM dogs require significantly more insulin per kg bw than T1DM humans. Since NIs can produce up to 1.2 U/kg bwt and are dosed on a per kg bw basis, this implies that NIs would be curative for T1DM in humans while not needing anti-rejection drugs, thereby readily exceeding the therapeutic potency of other current biotherapies.