SymbioCellTech’s Stem Cell-Enabled Therapy Could Offer a Functional Cure for Type 1 Diabetes Without Requiring Anti-Rejection Drugs
Under the leadership of stem cell experts Christof Westenfelder, MD, and Anna Gooch, PhD, SymbioCellTech is showing promising results on a T1D therapy involving “Neo-Islets.” They’ve cured T1D in mice, have had good results in dogs, and will begin human trials as soon as 2025.
The Challenge
When most people think about Type 1 diabetes (T1D), they think about a friend or family member who has to take shots of insulin in order to stay within a healthy blood sugar range. Or maybe you know someone who wears an insulin monitor on their arm and gets their blood sugar numbers sent to their phone. T1D is a devastating disease that affects millions of people, but thanks to the advent of new patches and pumps and apps, the public sees Type 1 diabetes as challenging but manageable.
For Anna Gooch, PhD, Type 1 diabetes conjures a more urgent picture, one that has driven decades of work in the lab and what could be a once-in-a-generation scientific breakthrough.
When Gooch thinks about T1D, she thinks about the patients her father took care of as a nephrologist. When she was as young as 10 years old he would take her on rounds with him as he cared for people with kidney disease.
“I got very attached to two patients in particular,” Gooch recalls. “They were both blind. My job was to sit and read out loud to them while they got their dialysis. They were bored to death.” They were also, as it turned out, living with diabetes.
One day she and her father arrived in the clinic to discover that one of these patients – who had recently had the good fortune of receiving a kidney transplant – was very sick. They had to rush him to the emergency department because his body was rejecting the transplant, and the anti-rejection drugs weren’t working.
“Transplants are great, but these drugs suck,” says Gooch. “You need something different. These drugs cause serious infections, cancer, and they destroy cells that are placed in the liver.”
That experience – both of caring for chronic disease patients and of seeing the body’s rejection of transplants – embedded itself in Gooch’s mind. She went on to get a PhD and spent 15 years working on cell therapy and regenerative medicine, often focusing on treatments for the same patent population that her father dedicated his life to.
Origin Story
Along her research journey Gooch found a kindred spirit in Christof Westenfelder, MD, a clinical nephrologist and expert in stem cell research. Westenfelder is an expert in regenerative medicine and previously founded Allocure. Together they began focusing their work on the problems inherent in certain diabetes treatments. Namely, the field of islet transplants. For years scientists have been honing this idea of injecting cells from a healthy pancreas into the body of a person with Type 1 diabetes, thus allowing them to produce insulin.
One major problem with typical islet cell transplants is that they require the life-long use of immunosuppressants that are often associated with infections, cancers, and kidney disease.
In 2014, the two came up with a hypothesis that, if correct, would change the course of diabetes treatment forever. They hypothesized that if they could work with the body’s natural functions, rather than against them, they could reduce islet cell therapy rejection, and make donor islet cells more readily available. It went like this:
“Islet cells in the pancreas contain small numbers of stem cells that monitor the health of the micro-environment continuously and help to fix minor problems,” explains Drs. Westenfelder and Gooch. “In a diabetic state, these cells get damaged and can no longer protect cells that produce insulin and other important hormones for metabolic control.” They hypothesized that if they increased the number of these protective stem cells they could help the insulin and other hormone-producing cells to survive and function. “These stem cells have a fantastic capacity to block the destruction of the cells that make insulin, which is what T1D is all about. We don't manipulate any of the cells, we just regroup them and reorganize them to work with each other, i.e., to collaborate with each other.”
Under the Hood
Drs. Gooch and Westenfelder set about doing what they did best: testing their hypothesis in a lab and publishing their research. At first, they had a healthy dose of scientific skepticism.
“This can't possibly work. How are you going to overcome the challenges of such a complex immune system?” Gooch recalls thinking in the early days.
Then came a day when that skepticism turned to hope.
They were in their lab in Salt Lake City, feeling “fairly jaded and cynical,” when they began to read the results of their first major mouse study. One after another they saw that each mouse, once fully diabetic, had normalized blood sugar. They had cured these mice of their diabetes, and they’d done it without harmful anti-rejection drugs.
The team moved from mice to dogs, which meant leaving the lab and testing their theories in the real world, with “patients” who didn’t always behave perfectly. Again, their results were incredibly encouraging.
“We had one diabetic dog who lived to be 17 years old,” says Dr. Gooch. “That’s astounding for a diabetic dog. They usually live about one year past diagnosis.”
Around 2017, the team published a detailed study of their “Neo-Islet” technology, explaining how it works, step by step. They also had earlier demonstrated their success through a clinical trial that showed that these stem cells could help patients undergoing cardiac surgery and patients that are at very high risk of losing their kidney function. The stem cells had a strong protecting effect for these patients, and now, 10 years post study, there's been no loss of kidney function, no deaths, no progression to chronic kidney disease compared to controls.
Next Steps
All eyes now are on human trials.
The SymbioCellTech team has had a successful pre-IND meeting with the FDA, which resulted in a request to do one more study using human cells, the final clinical product, in a suitable mouse model. Once they have their IND in hand, they’ll set up their human clinical trial, which they hope to have up and running in the second half of 2025.
SymbioCellTech has raised about $17M to date in support of this work, largely from friends, family, and angel investors passionate about curing Type 1 diabetes. The team regularly receives emails from families who hear about their work and want their family member to be part of a future clinical trial. For Drs. Westenfelder and Gooch, that’s something they take very seriously.
“You end up feeling responsible,” says Gooch. “You want to make sure that this works, is safe, is effective, is affordable, and can help them.”
SymbioCellTech has plenty of work to do, and questions to answer, before their hypothesis becomes a breakthrough for humans, but they’re already thinking about global impact.
“Once we show that it works in the US, we will already be well established in Europe with colleagues, scientists, and members of our board. We want to take this to the European market, but also to markets where there is a very limited amount of funding for this type of work.”
As with any scientific breakthrough, the proof will be in the data. We’re proud to support SymbioCellTech because they’ve formed a team with deep cell therapy expertise and their methodical progress is building towards a true breakthrough. If their human trials are successful, we could see a functional cure to Type 1 diabetes.
Connect with SymbioCellTech via email
SymbioCellTech Joins Startup Health’s Type 1 Diabetes Moonshot Fellowship to Advance Groundbreaking Neo-Islet Therapy
SymbioCelllTech becomes a member of StartupHealth’s Type 1 Diabetes Moonshot Fellowship
SymbioCelllTech is pleased to announce that the company has become a member of StartupHealth’s Type 1 Diabetes Moonshot Fellowship. The Fellowship, a community of more than 20 scientists, entrepreneurs and innovators, is one of the venture capital investment firm’s collaborative models to make progress on critical health challenges.
The Type 1 Diabetes Moonshot is supported by The Helmsley Charitable Trust and creates a three-year engagement for Fellows, working to address the 18 million-plus T1D patients around the world.
StartupHealth was launched in 2011 to focus resources on promising diagnostics, treatments and cures. The “moonshot” model imagines achieving “audacious health goals,” according to founder Steven Krein. “We see the potential in catalytic ideas and we work to bring together information, training, networks and resources so we can transform health challenges into successes.”
SymbioCellTech is developing “NeoIslets” (™), a new therapy that combines stem cells and pancreatic islet cells that enables the diabetic patient’s body to again produce adequate amounts of its own insulin, but without the need for toxic antirejection drugs or encapsulation devices. A cellular replacement therapy that works long term and doesn’t require anti-rejection drugs is a real breakthrough – it’s a therapy that all T1DM patients would be able to use.
“We are excited to welcome the SymbioCellTech team to the T1D Moonshot Community,” said StartUp Health’s Chief Strategy Officer, Bari Krein. “After meeting them in person at ADA this year, we knew they were aligned with our mission, and that of our T1D Moonshot. Their passion and commitment to working towards a cure is inspiring and we look forward to seeing how they grow with the support of our global founder community.”
“We’re pleased to be invited to join the Type 1 Diabetes Moonshot community,” said SymbioCellTech’s Dr. Anna Gooch, “because we recognize how important it is for all of us to work toward the shared goal of ending the diabetes spiral that affects so many people.” The company is in the process of its final phase of pre-clinical testing of its patented Neo-Islet(™) technology for its Investigational New Drug Application (IND) to be submitted to the FDA, “as soon as we can manage it,” according to Dr. Gooch. With the IND, SymbioCellTech plans to conduct the First-in-Human Clinical Trial likely at the City of Hope, UC San Diego, UCLA and UC Davis
NeoIslets: Innovation Challenge Winner at ADA Scientific Session
SymbioCellTech's Innovative Diabetes Therapy Gets Us One Step Closer to Effective, Easily Tolerated Treatment of Type 1 Diabetes
NeoIslets” (™) work without the lifelong need for toxic anti-rejection drugs, which is one feature that distinguishes this therapy from other cellular replacement therapies”
— Dr. Anna Gooch
SALT LAKE CITY, UTAH, USA, August 13, 2024 /EINPresswire.com/ -- Researchers are one step closer to a cure for Type 1 diabetes as demonstrated by one winner of the “Innovation Challenge” award at this year’s American Diabetes Associations’ Scientific Meeting in Orlando, FL. The ADA’s Innovation Challenge is designed to further “transformative solutions for people living with diabetes, their families, and caregivers,” and SymbioCellTech, a Salt Lake City-based regenerative medicine company was one of only 3 winners honored with this award at this year’s meeting.
SymbioCellTech has developed “NeoIslets” (™), a new therapy that combines stem cells and pancreatic islet cells that enables the diabetic patient’s body to again produce adequate amounts of its own insulin. The procedure has been tested in nine autoimmune, diabetic dogs over a three-year period, and all nine dogs showed dramatic and lasting improvement in markers of Type 1 diabetes. Importantly, the therapy appeared safe and well tolerated, resulting in no significant changes in blood chemistries or cells counts. Furthermore, the usual organ damage seen in even some well-controlled diabetic patients, to kidneys, hearts, and nerves, for example, were not seen in these dog patients: the therapy appeared to help preserve renal function.
“What’s very exciting is the news that “NeoIslets” (™) work without the lifelong need for toxic antirejection drugs, which is one feature that distinguishes this therapy from other cellular replacement therapies” said SymbioCellTech’s Drs. Anna Gooch and Christof Westenfelder, the company’s lead researchers, who presented these findings at the ADA. While Antirejection drugs are required to prevent a patient from rejecting cells or organs that come from an unrelated donor, they come with many toxic and sometimes life-threatening side effects, which means that most Type 1 Diabetic (T1DM) patients cannot take advantage of the cellular therapies that are available. The risks of the antirejection drugs in most cases do not outweigh the benefits. A cellular replacement therapy that works long term and doesn’t require anti-rejection drugs is a real breakthrough – it’s a therapy that all T1DM patients would be able to use. “Because dogs with Type 1 diabetes are physiologically similar to humans, but typically require significantly more insulin than humans, we’re confident that the successful dog trials open the door to human trials and a fresh start for millions of diabetics.”
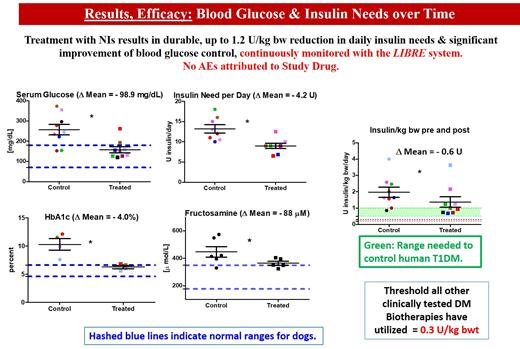
Dr. Gooch underscored that all dogs completed the trial “without any impairment or diabetes-related negative reactions,” and that daily insulin doses were reduced an average of 0.85 and up to as much as 1.2 Units of insulin per kg body weight per day. Simultaneously, serum glucose levels (reported as mg/dL, or milligrams per one-tenth of a liter) dropped by nearly 100 mg/dL, and elevated Hemoglobin A1C levels also fell to normal by an average of 4%.
Dr. Westenfelder pointed out that the “NeoIslets” (™) technology helps to overcome the serious shortage of donor organs, making it possible to greatly multiply the therapeutic value of a single donated pancreas. “This success with dogs points the way to effective, efficient, affordable and safe therapies for humans,” he added.
SymbioCellTech is in the process of its next phase of the development of the patented “NeoIslets” (™) technology for an Investigational New Drug Application (IND) from the FDA, “as soon as we can manage it,” according to Dr. Gooch. With the IND, SymbioCellTech plans to conduct the First-in-Human Clinical Trial likely at the City of Hope, UC San Diego, UCLA and UC Davis.
_________________________
ABOUT SYMBIOCELLTECH:
SymbioCellTech (SCT) is a late preclinical stage Regenerative Medicine Company based in Salt Lake City, UT. The company has created a novel NEO-ISLETS(TM) technology that is readily scalable and that will reduce, and potentially eliminate, the daily insulin injection needs of people with Type I Diabetes mellitus. The NEO-ISLET(TM) technology has demonstrated its safety and efficacy in an Investigational New Animal Drug study in diabetic dogs and offers the potential to significantly increase available doses, eliminate the need for anti-rejection drugs and is a permanent solution for diabetics.
DISCLAIMER: FORWARD-LOOKING STATEMENTS
This press release contains forward-looking statements including (a) statements by Anna Gooch, Ph.D., and Christof Westenfelder, M.D., in this press release, (b) our plans, expectations for, and the potential benefits of NEO-ISLETS(™), and (c) our plans for additional research. While SymbioCellTech believes the forward-looking statements contained in this press release are accurate, these forward-looking statements represent the company's beliefs as of this press release. Risks and uncertainties could cause actual events or results to differ materially from those expressed or implied by such forward-looking statements. Those risks and uncertainties include, among other things, that these data may not be indicative of final clinical trial results, that data from the company's research and development programs may not support further development of its products due to safety, efficacy, and other risks.
SymbioCellTech Wins ADA Innovation Challenge for Groundbreaking Diabetes Biotherapy
The American Diabetes Association® (ADA) announced the winners of its prestigious Innovation Challenge competition. Innovators from across the globe presented groundbreaking ideas aimed at transforming diabetes care before a distinguished panel of potential funders and an audience of professionals in the field. The objective was clear: to advance transformative solutions for people living with diabetes, their families, and caregivers. Among the three winners selected from five finalists presenting at the ADA’s 84th Scientific Sessions, SymbioCellTech emerged as a winner in the challenge.
SymbioCellTech’s innovative biotherapy for patients with type 1 diabetes stands out for its unique approach that eliminates the need for encapsulation devices or antirejection drugs. This remarkable advancement promises to significantly improve the quality of life and is recognized as a highly anticipated therapeutic to take into human trails.
About the American Diabetes Association’s Scientific Sessions
The ADA's 84th Scientific Sessions, the world's largest scientific meeting focused on diabetes research, prevention, and care, will be held in Orlando, FL on June 21-24. More than 11,000 leading physicians, scientists, and health care professionals from around the world are expected to convene both in person and virtually to unveil cutting-edge research, treatment recommendations, and advances toward a cure for diabetes. Attendees will receive exclusive access to thousands of original research presentations and take part in provocative and engaging exchanges with leading diabetes experts. Join the Scientific Sessions conversation on social media using #ADAScientificSessions.
SymbioCellTech's CSO Anna Gooch, PhD, to Present on Neo-Islet™ Technology at ADA 84th Scientific Sessions
Anna Gooch, PhD, will deliver an oral presentation on Neo-Islet™ technology at the American Diabetes Association's 84th Scientific Sessions on Sunday, June 23, 2024. Her presentation, titled "334-OR: Allogeneic Neo-Islets (NIs) Reduce Insulin Need by up to 1.2 U/kg bw in Autoimmune Type 1 Diabetes Dogs over Three Years without Requiring Antirejection Drugs, Enough to Meet Human Type 1 Diabetes Mellitus Needs," will include a Q&A session for attendees.
The spontaneously diabetic dog is ideal to test therapies intended for humans with T1DM. Neither insulin treated dogs nor humans spontaneously revert. Both have islet autoantibodies, shortened life expectancy, end-organ damage, while dogs need more insulin (humans: 0.5-1; dogs: 1-4 U/kg/day). We tested Neo Islets (NIs), 3-D organoids made of culture expanded islet cells and Mesenchymal Stem Cells in 9 spontaneously DM pet dogs. NI dose = 2x10e5 per kg bw, given i.p. to sedated dogs under ultrasound guidance. Follow up: Blood Glucose Levels monitored with continuous monitors. Insulin needs were recorded, and late follow-up levels of serum Creatinine, BUN and proteinuria were monitored. Each dog served as its own control. UA, full chemistries, lipids, CBC were assessed at baseline, monthly for 3 months, and biannually for 3 years. Results in Figure.
Allogeneic NI treatment is safe and effective (3 year follow up). T1DM dogs require significantly more insulin per kg bw than T1DM humans. Since NIs can produce up to 1.2 U/kg bwt and are dosed on a per kg bw basis, this implies that NIs would be curative for T1DM in humans while not needing anti-rejection drugs, thereby readily exceeding the therapeutic potency of other current biotherapies.
SymbioCellTech, LLC Honored as Runner-Up in Medical & Health Med Tech at the 2023 Innovation Awards
SymbioCellTech, LLC has been named runner-up in the Medical & Health Med Tech category at the 2023 Innovation Awards. This prestigious accolade celebrates the innovative spirit of Utah companies and the talented individuals dedicated to solving complex problems with elegant solutions.
Our recognition underscores our commitment to advancing healthcare and our groundbreaking contributions to the field.
Novel Biotherapy for Type I Diabetes Presented at This Year’s American Diabetes Association Meeting
SymbioCellTech’s “Neo-Islet” technology, a minimally invasive, economical, and safe Stem Cell-enabled functional Cure for T1DM without immunosuppressive drugs.
the process of creating Neo-Islets (™) is efficient, and we’ve demonstrated that we can produce what patients require”
— Anna Gooch, PhD
SALT LAKE CITY, UT, USA, September 6, 2023/EINPresswire.com/ -- SymbioCellTech (SCT), the regenerative medicine company, has released results of athree-year Investigational New Animal Drug (INAD) studythat underscore the safety, effectiveness and feasibility of using Neo-Islets(™) in treating diabetic dogs, and offering such dog owners some hope for their pets. While this version of NI therapy is specifically formulated for dogs, the promising results from this dog study have positive and important implications for upcoming human trials which will test a human formulation of Neo-Islets(™).
Under the guidance of the U.S. Food and Drug Administration, SCT researchers began treatment of nine dogs (Bichon mix, Chihuahua mix and Jack Russell terriers), each diabetic and each dependent on insulin. The company administered Neo-Islets(™), a cellular replacement therapy that reestablishes adequate production of insulin. The INAD study focused on three objectives and “we met all our endpoints,” according to SCT chief science officer Dr. Anna Gooch.
The impact of Neo-Islet(™) therapy is durable – six of the dogs in the study are now three years past treatment, the other three are still within the study protocols.
The primary concern was the safety of Neo-Islet(™) therapy. There were no serious adverse events related to the use of Neo-Islets in the study, nor was there any serious deterioration in liver function, lipids, electrolytes, renal function (kidney function) or blood cell counts.
In addition to safety, SCT sought to determine whether or not Neo-Islets(™) therapy is a feasible approach. In the study, 12 therapeutic doses were produced and that yield was sufficient to treat all nine dogs, with four of them receiving a second dose and one a double dose. Dr. Gooch pointed out that “the process of creating Neo-Islets (™) is efficient, and we’ve demonstrated that we can produce what patients require.”
“What’s really promising is that these dog studies offered further evidence, and in a larger mammal, of the effectiveness of Neo-Islets(™),” according to SCT founder and CEO Dr. Christof Westenfelder. Serum glucose levels, the measure of blood sugar that reflects whether or not a patient is diabetic, fell to about 99 milligrams per deciliter, the normal range, and HbA1C levels dropped an average of four percent, also into the range considered normal for these dogs.
In addition to the observed better blood sugar control, dogs’ need for insulin dropped significantly over the treatment period. Dogs tend to need more insulin than humans, so the impact of Neo-Islet™ therapy on dog diabetes suggests that it can be even more impactful on humans. “We did this without any evidence of disease progression, or any damage to major organs,” Westenfelder added.
This third endpoint of the study stands out because it was accomplished without the use of any anti-rejection drugs, which are a burdensome, potentially harmful, and a problematic requirement of most other, otherwise promising diabetes biotherapies.
Dr. Gooch summed up the results of the INAD study with a brief acknowledgement of the work still ahead and a glimpse of its potential.
“We will continue to improve the efficacy of Neo-Islet(™) therapy in dogs by working on modifying the dose, adjusting its rate of achieving maximum potency and taking some other steps to make it even more useful in treating T1DM. With these very positive results treating diabetic dogs, and given the lower insulin requirements in Type 1 Diabetic humans compared to dogs, we are very hopeful about the implications this study has for people, and we are moving closer to the FDA-directed trials in humans, a real breakthrough for millions of diabetics.”
ABOUT SYMBIOCELLTECH:
SymbioCellTech (SCT) is a late preclinical stage Regenerative Medicine Company based in Salt Lake City, UT. The company has created a novel NEO-ISLETS(TM) technology that is readily scalable and that will reduce, and potentially eliminate, the daily insulin injection needs of people with Type I Diabetes mellitus. The NEO-ISLET(TM) technology has demonstrated its safety and efficacy in an Investigational New Animal Drug study in diabetic dogs and offers the potential to significantly increase available doses, eliminate the need for anti-rejection drugs and is a permanent solution for diabetics.
DISCLAIMER: FORWARD-LOOKING STATEMENTS
This press release contains forward-looking statements including (a) statements by Anna Gooch, Ph.D., and Christof Westenfelder, M.D., in this press release, (b) our plans, expectations for, and the potential benefits of NEO-ISLETS(™), and (c) our plans for additional research. While SymbioCellTech believes the forward-looking statements contained in this press release are accurate, these forward-looking statements represent the company's beliefs as of this press release. Risks and uncertainties could cause actual events or results to differ materially from those expressed or implied by such forward-looking statements. Those risks and uncertainties include, among other things, that these data may not be indicative of final clinical trial results, that data from the company's research and development programs may not support further development of its products due to safety, efficacy, and other risks.
Novel Biotherapy for Type I Diabetes Presented at This Year’s American Diabetes Association Meeting
SymbioCellTech Shares Breakthrough Data on Impact of Novel Human NEO-ISLETS(TM) in the Efficient Utilization of Currently Discarded Tissues
We have conducted three proof-of-concept studies in two species, and the positive results mean that this therapy can be refined for the treatment of human T1DM.”
— Anna Gooch, PhD
SALT LAKE CITY, UT, USA, August 24, 2023/EINPresswire.com/ -- BACKGROUND: DIABETES TREATMENT BEYOND INSULIN
Insulin is a hormone that allows the body to deliver sugar to the cells which they use for energy. Type 1 Diabetes Mellitus (T1DM) is a disorder in which the insulin producing cells in the pancreas are attacked and killed by the patient’s own immune system.. Without this ability, even though the person eats sugar, it cannot get to the cells, but remains in the blood, and the cells starve and die. For T1DM patients, insulin injections are life-saving, but cannot prevent the development of serious diabetic complications such as blindness, kidney failure, strokes, heart attacks, amputations, premature births and deaths.
For decades, researchers have been trying to find a way to naturally replace the destroyed insulin producing cells found within islets in the pancreas. The treatment of T1DM with transplanted organs (pancreases) or cells from those organs dates to the mid-1960s, but donor organs are scarce, and frequently, organs or cells are unsuitable for transplant due to suboptimal conditions of the tissues, and the donated organs are discarded. Another significant limitation to many cell replacement strategies is that they require the lifelong use of potentially harmful anti-rejection drugs. Both these drawbacks severely limit the availability of these therapies to patients.
The Regenerative Medicine company SymbioCellTech may have found a highly effective solution to overcoming those challenges.
In an article for the August 24 issue of PLOS ONE, SymbioCellTech (SCT) reports significant success using human Neo-Islets created from suboptimal donors and donor tissues in the treatment of T1DM.
The paper, published today in PLOS ONE, is “Significant Expansion of the Donor Pool Achieved by Utilizing Islets of Variable Quality in the Production of Allogenic “Neo-Islets,” 3-D Organoids of Mesenchymal Stromal and Islet Cells, A Novel Immune-Isolating Biotherapy for Type 1 Diabetes” authored by Anna M. Gooch, PhD, Sabiha S. Chowdhury, PhD, Ping M. Zhang, MD, Zhuma M. Hu, MS, and Christof Westenfelder, MD, FACP.
Dr. Westenfelder, Founder and CEO of SymbioCellTech, said “In our research, we wanted to demonstrate that donor islets judged unsuitable for clinical islet transplantation - applying the rigorous NAIDS scoring system - could be used to manufacture NEO-ISLETS(TM) and restore euglycemia and insulin-independence. Our report clearly demonstrates that this has been successfully achieved.”
Neo-Islets(™) are a cellular replacement therapy that work, in part, by culture expanding islet from donated pancreases. This culture expansion results in more or less uniform quality of cells at the end, and allows for the use of tissue and cells that, while not suitable for transplant as they are, work and are suitable for this application. Combination of these cells into Neo-Islets ™ with another cell type that normally modulates the immune system makes it possible to use the therapy without immunosuppressive drugs or encapsulation devices.
Indeed, earlier published studies from SCT demonstrate that NEO-ISLETS™ provide durable normalization or reduction in blood sugar levels and insulin-independence or a significant reduction in the use of insulin, and that the impact of NEO-ISLETS(TM) has been achieved without the lifelong use of potentially toxic and expensive anti-rejection drugs. This was demonstrated in mice, and in an extensive, 3 year study, in pet dogs with diabetes.
This latest research demonstrates that successful production of NEO-ISLETS(TM) is accomplished regardless of demographic and quality differences in donor tissues.
Dr. Gooch, SCT’s Chief Science Officer and the lead author of the PLOS ONE paper, emphasized the implications of the findings: “We have conducted three proof-of-concept studies in two species, and the positive results mean that this therapy can be refined for the treatment of human T1DM. Like islet and pancreas transplants, NEO- ISLET(TM) therapy relies on islet donors, but to a much smaller extent due to the use of culture expanded islet cells rather than the islets themselves.”
SCT is actively pursuing the next stage of NEO-ISLET(TM) development with the conduct of an IND-enabling Proof of Concept study that will move the therapy to human trials.
FULL ARTICLE HERE:https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0290460
___________________
ABOUT SYMBIOCELLTECH:
SymbioCellTech (SCT) is a late preclinical stage Regenerative Medicine Company based in Salt Lake City, UT. The company has created a novel NEO-ISLETS(TM) technology that is readily scalable and that will reduce, and potentially eliminate, the daily insulin injection needs of people with Type I Diabetes mellitus. The NEO-ISLET(TM) technology has demonstrated its safety and efficacy in an Investigational New Animal Drug study in diabetic dogs and offers the potential to significantly increase available doses, eliminate the need for anti-rejection drugs and is a permanent solution for diabetics.
DISCLAIMER: FORWARD-LOOKING STATEMENTS
This press release contains forward-looking statements including (a) statements by Anna Gooch, Ph.D., and Christof Westenfelder, M.D., in this press release, (b) our plans, expectations for, and the potential benefits of NEO-ISLETS(™), and (c) our plans for additional research. While SymbioCellTech believes the forward-looking statements contained in this press release are accurate, these forward-looking statements represent the company's beliefs as of this press release. Risks and uncertainties could cause actual events or results to differ materially from those expressed or implied by such forward-looking statements. Those risks and uncertainties include, among other things, that these data may not be indicative of final clinical trial results, that data from the company's research and development programs may not support further development of its products due to safety, efficacy, and other risks.
SymbioCellTech Demonstrates Preclinical Efficacy of Proprietary and Novel Human Neo-Islets™ Technology in STZ-Diabetic NOD-SCID Mice
Clinically relevant model validates Neo-Islets™ as a durable and potentially curative therapy for Type 1 Diabetes that does not require toxic anti-rejection drugs
SALT LAKE CITY, Oct. 28, 2021 (GLOBE NEWSWIRE) -- SymbioCellTech (SCT) announced today the publication of a paper demonstrating the potency and efficacy of its innovative and proprietary human Neo-Islet™ in a third, clinically relevant model of human Type 1 Diabetes (T1D). Published in PLOS ONE, the paper describes SCT's creation of a "new endocrine pancreas" that delivers islet hormones into the hepatic portal vein. This is the only non-transplant therapy to provide physiologic delivery of insulin and other islet hormones without the need for non-cellular encapsulation devices or anti-rejection drugs.
Christof Westenfelder, MD, founder and CEO of SCT, stated, "We continue to advance our durable cure for those suffering from Type 1 Diabetes towards investigation in a Phase I Clinical trial and the curative effect of human Neo-Islets™ in STZ-diabetic NOD/SCID mice adds further support to our translational expectations of the therapy." Westenfelder added, "We're excited to bring forward a durable mechanism of insulin delivery through particular insights into Mesenchymal Stromal/Stem Cells, the most exquisite of encapsulation technologies." The design of Neo-Islets™ provides permanent auto-immune and allo-immune isolation, a critical activity that most other encapsulation technologies lack because they induce a foreign body reaction that leads to failure of the transplant.
Previously, in an article published in Stem Cells Translational Medicine, the SCT team demonstrated allogeneic Neo-Islets™ (three-dimensional organoids composed of approximately equal numbers of Mesenchymal Stromal Cells and culture-expanded Islet Cells) permanently restore blood sugar levels to normal in diabetic NOD mice after spontaneous engraftment of Neo-Islets™ into the omentum. Furthermore, in an article published in PLOS ONE, treatment of insulin-dependent pet dogs with allogeneic canine Neo-Islets™ (ongoing FDA-INAD Pilot Study being conducted in a clinical setting) showed stable and durable statistically significant reduction in need for insulin, as well as consistently improved glycemic control without antirejection drugs.
Anna Gooch, PhD, Chief Scientific Officer, commented, "The fact that our human Neo-Islet™ has a similar gene expression profile to that of our dog and mouse Neo-Islets™ further suggests this therapy holds promise for successful translation to the clinic."
This content was issued through the press release distribution service at Newswire.com.